What is ARIC Generation 2?
The purpose of the ARIC Gen2 research study is to evaluate the link between glucose and heart problems in adults with type 2 diabetes. Heart problems can be common in people with type 2 diabetes. We are interested in measuring your blood sugar (glucose) using a continuous glucose monitor and monitoring your heart rhythm at the same time.

Is ARIC Gen2 for me?
Participants will be adults aged 50 to 80 years of age with a diagnosis of type 2 diabetes. If you have type 2 diabetes, you are potentially eligible to participate in our study.
What will happen?
Participants will be asked to come to the ARIC Field Center to collect some information and we will also study your glucose and heart rhythm for 2 weeks following the clinic visit.
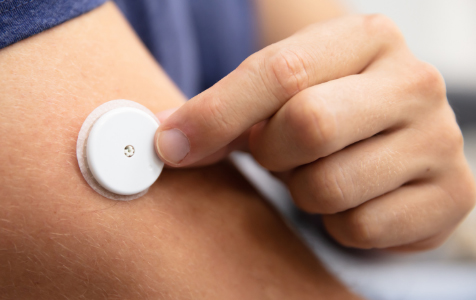
Your glucose and heart rhythm will be monitored using two separate devices that are very small and placed on the skin. The glucose monitor is placed on the arm. The heart rhythm monitor is a sticker that is placed on your chest. Both devices are regularly used by medical doctors. They are worn for two weeks.
- During the initial clinic visit, participants will be asked some questions to collect basic information such as medical history, physical activity, and medication use. We will also get your blood pressure, blood sample, height, weight, physical function and do some memory tests.
- The heart rhythm monitor is a sticker that is placed on your chest.
- The continuous glucose monitor (CGM) is placed on the arm.
Will I be compensated?
Participants will receive a payment for participation up to $165. Free parking and snacks will be provided at the clinic visit.
Study Duration
Participation in the research study will be for two weeks with additional follow-up. The two weeks includes the initial visit to the Field Center, wearing the devices, and then mailing the devices back after the two-week monitoring period is over.
Participants will also be followed over time with semi-annual phone calls and hospital chart review.
Study Site Locations:
Johns Hopkins University
George W Comstock Center for Public Health Research and Prevention
1100 Dual Highway, Hagerstown, MD 21740
Phone: 301-791-1847
Epidemiology Clinical Research Center
University of Minnesota
1100 Washington Ave S, Suite 201
Minneapolis, MN 55415
Phone: 612-624-8914
Wake Forest University School of Medicine
Study Contact Name: Carol Thomas
Piedmont Plaza 1 Suite 101
1920 West First Street
Winston-Salem, NC 27104
Phone: 336-716-5742
University of Mississippi Medical Center
2500 North State Street
Office Annex 2, ND 342
Jackson, MS 39216
Phone: 601-984-5695
Study Number: IRB00311999
Phone: 410-955-0495
Dr. Josef Coresh, Principal Investigator
